Did the Oxford-AstraZeneca vaccine really only decrease the risk of covid by 1.2%?
A multi-million pound claim in the high court suggests it did.

A multi-million pound landmark “vaccine damage” case is set to take place in the high court. The test case is being pursued by Jamie Scott who suffered a severe brain injury in April 2021 after receiving the Oxford-AstraZeneca vaccine.
The case being brought under the Consumer Protections Act argues that the Oxford-AstraZeneca vaccine was less safe than consumers were entitled to expect. A key part of the argument is over the efficacy1 of the vaccine, which claimants argue was “vastly overstated”.
What are the risks?
To get into the nitty-gritty of the claim we need to understand something about how risks are presented. There are two different ways to present the change in risk brought about by a treatment or an intervention: absolute risk and relative risk. Lets have a look at an example from my first book – The Maths of Life and Death - to explain the difference.
In 2009, under the headline ‘Careless pork costs lives’, the Sun reported just one of many hundreds of results from a 500-page study by the World Cancer Research Fund, on the effect of consuming 50 grams of processed meat per day. The tabloid newspaper shocked readers with the ‘fact’ that eating a bacon sandwich every day would increase the risk of colorectal cancer by 20%.
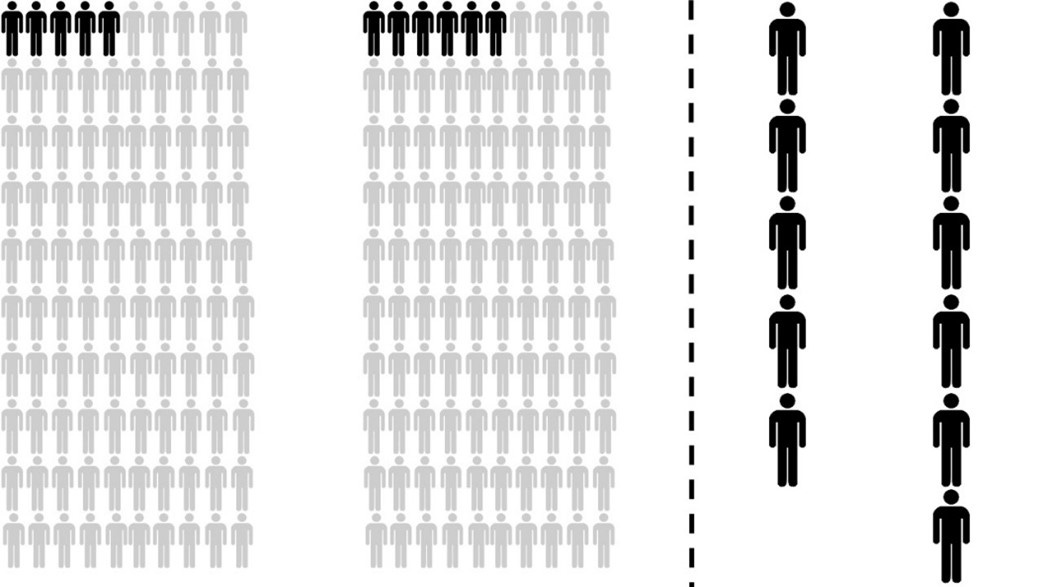
When stated in terms of ‘absolute risks’ – the proportion of people exposed or unexposed to a particular risk factor (e.g. eating bacon sandwiches or not eating bacon sandwiches) who are expected to develop a given outcome (e.g. cancer) in each case – it turns out that 50g of processed meat per day increases the absolute lifetime risk of developing colorectal cancer from 5% to 6%. On the left of Figure 1 we consider the fates of two groups of 100 individuals. Of 100 people who eat a bacon sandwich every day, only one more of them will develop colorectal cancer than in a group of 100 people who abstain.
Figure 1: A comparison of the absolute figures (5 in 100 vs 6 in 100) (left) makes it appear that the increase in risk of eating 50g of processed meat per day is small. By focussing on the relatively small number of people who have the disease (right) the relative risk increase of 20% (1 in 5) seems very large.
The Sun chose to focus on the ‘relative risk’ – the risk of a particular outcome (e.g. developing cancer) for people exposed to a given risk factor (e.g. eating bacon sandwiches) as a proportion of the risk for the general population. If the relative risk is above 1 then an exposed individual is more likely to develop the disease when compared to someone without the exposure. If it is below 1 then the risk is decreased.
On the right hand side of Figure 1, by neglecting the people who are not affected by the disease, the increase in the relative risk (6/5 or, equivalently, 1.2) of 20% seems much more dramatic. So it is true that the relative risk for those eating 50g of processed meat per day represents an increase of 20%, but the absolute risk increased by only 1%.
The claimants in the court case argue that in a press release about the vaccine, AstraZeneca point to studies showing around 70% efficacy at preventing symptomatic covid and 90% under a different dosing regimen. As you can see from the excerpt from the court documents below, the legal claim states “In fact, the absolute risk reduction concerning Covid-19 prevention was only 1.2 per cent.” The basis of the case that the efficacy of the vaccine was overstated seems to be, like the Sun’s “save our bacon” campaign, that AstraZeneca had inflated the apparent efficacy of their vaccine by using relative risk rather than absolute risk.
Figure 2: Excerpt from the legal case filed with the high court in which the argument about absolute vs relative risk presentations are made.
There are good reasons why AstraZeneca would have used the relative risk reduction rather than the absolute for describing the effectiveness of their vaccine. For one, the prevalence of covid varied significantly throughout the acute phase of the pandemic. When the prevalence was low then the absolute reduction in risk would necessarily be lower than when the prevalence was high.
Imagine a hypothetical vaccine that blocked half of all infections in two different prevalence scenarios – one at 10% and another at 1% prevalence. In the first scenario the absolute risk reduction from taking the vaccine would be 5%, but in the second it would be 0.5%, whereas the relative risk reduction would always be 50%. In the face of changing prevalence, it makes sense to use the relative risk reduction to explain how much safer the vaccine makes you.
Another good reason for using relative effectiveness hinges on understanding the way in which clinical trials are often run. Typically, trial volunteers are split into a treatment group – who are given the vaccine - and a control group who are not (although they are sometimes given a placebo treatment). When running a trial, partly for time constraints and partly because it is unethical to let lots of unvaccinated people get infected, a limit is set on the proportion of people in the unvaccinated group who are allowed to be infected. Once this limit is reached, the trial is halted and the comparison between the proportion of infections in the control group and the treatment group can be made to assess the effectiveness of the vaccine.
Imagine, in a hypothetical trial, that this limit were set at 2% and that when the trial was halted only 0.2% of the treatment group had been infected. This would give a crude estimate of the relative efficacy of the vaccine of 90%, but an absolute efficacy of only 1.8%. Even with a vaccine which blocked all infections it would never be possible to demonstrate an absolute efficacy greater than 2% because of the set-up of the trial.
Given that the ONS suggests that we have now had enough infections in the UK for everyone to have been infected with covid at least once, using the relative efficacy seems like a sensible strategy. Is it reasonable to suggest that statements of the efficacy of vaccines should be limited because of the trial design or the fluctuating prevalence of a disease? It will be interesting to see how the arguments play out in court. Irrespective of what the court rules, what is not in doubt, despite some setbacks, is that the Oxford-AstraZeneca vaccine saved millions of lives.
Efficacy is the extent to which a vaccine is able to prevent a disease (or transmission) under the controlled conditions of a clinical trial. Effectiveness is an indicator of the same capabilities, but in real-world circumstances. I will try to be accurate in my usage but sometimes the words will be used interchangeably. I have also used the catch-all term “risk reduction” in some places in stead of efficacy or effectiveness.